
Newsletter
The College of Reproductive Biology (CRB) is a not-for-profit special interest group of the American Association of Bioanalysts (AAB).
Digital QA – The Future of ART Lab Management
Carol Lynn Curchoe Burton, Ph.D., TS(ABB)
Staff competency and related metrics are the number one most cited IVF laboratory inspection deficiency. Embryology and andrology procedures are subjective, complex, and difficult to standardize. The lack of rigorously standardized laboratory protocols and strict quality control (QC) confounds even the best laboratories. Easily ensuring compliance with LQMS, CLIA, and WHO standards is an invaluable tool for clinics and laboratory directors. Accurate laboratory test results depend on staff being competent to perform a range of procedures and competency assessments are part of a laboratory’s quality documents, and should be periodically reviewed and used for continuous improvement.
Mobile application technology has recently been applied to the field of medical research. Over 165,000 health-related applications now improve health, facilitate clinical trials research, and most recently, integrate with sophisticated external hardware to deliver point-of-care diagnostic assays. Mobile application technology allows for rapid data collection and real time reporting, real-time analysis, management and distribution of multimedia files, and the ability to utilize hardware add-ons or proprietary device hardware features (gyroscope, microphone etc.), and to collect biometric (fingerprint) data to enhance security. The ability to implement compliance standards ensures that tester data can be reported electronically to a central agency without compromising privacy standards or sacrificing efficiency.
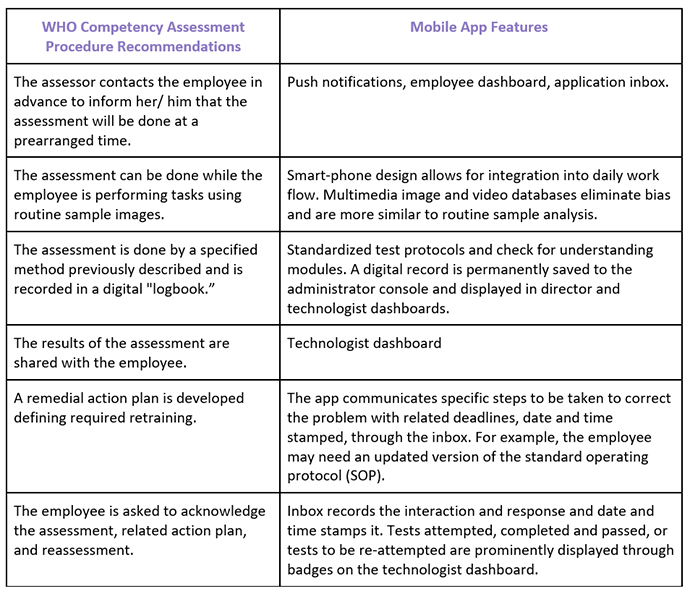
Mobile applications are providing HIPAA-compliant ways to assess the clinical decision making of ART laboratory staff for andrology and embryology competency. The competency assessment modules provide standardized instructions to test-takers and can be used to measure inter and intra- technologist variability between embryologists. Competency assessment surveys have been designed to allow the ART/ IVF laboratory director to gain insight into the clinical decision making of the most senior staff and compare that to junior staff members. For example, for choice of sperm for ICSI, or top choice of embryo for cryopreservation, biopsy, and transfer, and inform the key performance indicators (KPIs) used to continuously monitor and assess culture conditions. Mobile application technology was designed to allow standardized specimens to be served to each technologist at each study site simultaneously, allowing even very small IVF clinics to compare an individual technician’s values to the mean of all technicians and to technicians in a central laboratory. Test pictures, videos, and written test questions are randomly refreshed from a large database of multimedia files to eliminate bias.
Current assessments methodologies are extremely limited, perhaps to just one cleavage stage embryo and one blastocyst image every 6 months, and they cannot be customized to a lab’s own grading system or clinical question(s) of interest to that particular lab. Mobile app assessments are unlimited and completely customizable- from the images to the buttons to the test directions and the pre-test video can deliver learning content or instructions and demonstrations.
The mobile app documents ALL aspects of laboratory information assessment, not just for embryos and sperm but including; basic lab, continuing education, biohazard safety, handwashing, color vision, and FDA regulations among many others. ART Compass provides standard forms used by all employees. It documents competency assessment records, time and date stamps results, and is completely confidential. These records become part of the laboratory’s quality documents, and can be periodically reviewed and used for continuous improvement and quality assurance.
Additionally, pictures, videos, and written test questions are randomly refreshed every month from a large database of multimedia files to eliminate bias (being familiar with images and expected answers for example) for an ART laboratory’s quality assurance plan.
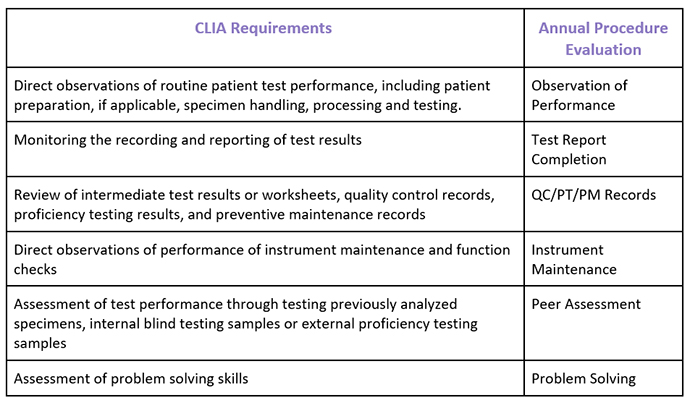
Evaluating and documenting competency of personnel responsible for testing is required at least semiannually during the first year the individual tests patient specimens, and at least annually thereafter. Competency assessment must be performed for testing personnel for each test that the individual is approved by the laboratory director to perform. The following six (6) procedures are the minimal CLIA regulatory requirements for assessment of competency for all personnel performing laboratory testing.
Disclaimer: The content and views presented in this article are those of the author and do not necessarily reflect those of the American Association of Bioanalysts (AAB) or the AAB College of Reproductive Biology (CRB). The information is not necessarily exhaustive of the subject matter, and laboratory professionals and all other individuals should review and consider other relevant materials, and professional opinions and advice, on the subject matter before making appropriate decisions.
Board Certification for Andrology and Embryology Laboratory Professionals ⇒CRB News Articles
06/24/2020
Vol 10, No. 1
06/24/2021
From The President
06/24/2021
Meeting Committee
06/24/2021
Legislative Committee
06/24/2021
Credentialing and Membership Committee - Survey on Impact of COVID-19
06/24/2021
Publication Committee
06/24/2021
2021-2022 CRB Officers and Board Members
06/24/2021
Earn ABB/PEER CE Credit Attending Select 2021 ASRM Virtual Post-Graduate Courses
06/24/2021
2022 CRB Symposium, May 11-14
06/24/2021
Preventing Cryostorage Failure: What Have We Learned?
06/24/2021
Digital QA - The Future of ART Lab Management
06/24/2021
Board Certification for Andrology and Embryology Laboratory Professionals
CRB Standing Rules - Log in to view



