
Newsletter
The College of Reproductive Biology (CRB) is a not-for-profit special interest group of the American Association of Bioanalysts (AAB).
Preventing Cryostorage Failure: What Have We Learned?
Mitchel C. Schiewe, Ph.D., HCLD/ELD(ABB)
Over the past couple years, the AAB/CRB has reminded us of the importance of quality management in our ART laboratories, particularly as it has pertained to cryostorage, witnessing events and the legal ramifications of laboratory incidents. Surprisingly, it has not been uncommon to hear about the occasional wrongful insemination or accidental embryo misuse that has occurred. As shocking as it is to hear such news, beyond the empathy we experience for the patients involved, our most pronounced feeling is one of great relief. “Thank goodness that wasn’t our lab." These events remind us of the importance of diligent witnessing of key gamete/embryo handling steps, attention to detail, honesty and open communications. Yet, it was in the winter of 2018 that all of us were dramatically impacted by a single event, the press release involving a catastrophic failure of a high capacity bulk tank containing thousands of sperm, oocyte and embryo samples. The fear that such an event could occur in any of our laboratories was indeed a humbling thought.
The 2018 mass media release of a second potentially significant tank failure in another part of the country was a wake call for all of us. Interestingly, these publicly exposed events were not isolated incidents, in fact hundreds other storage failure events involving gametes and embryos have occurred (Tomlinson & Morroll, 2008; Moutos et al., 2019), the severity of which is often silenced by legal non-disclosure agreements. And still many others have occurred, but disastrous outcomes were effectively mitigated by prompt action, good quality control or dumb luck (i.e., tank not in use). A recent CryoPort sponsored webinar (July 2018) revealed that over 50% of the 70+ participants had experienced some form of failure, most often involving dry-shipper tanks. Dry-shipper tanks may be exposed to uncontrolled conditions enroute to a destination, warranting the use of GPS trackers, temperature data loggers and level/tip indicators. Routine weighing and LN2 filling before and after shipments is standard practice in cryobanks to assess tank performance and track historical evaporation rates to anticipate retirement before a problem occurs.
Meanwhile, storage tank QC can be a painstakingly boring process due to its incredibly mundane measures and consistent outcomes. The latter is especially true in the small dewar storage systems (<73L; typically 35-45L) without any automation or electrical dependency. Indeed, our basic tank systems have been very consistent over time, requiring nothing more than a weekly refilling practice to insure decades of reliable usage. Thus, it is not surprising that many of us in IVF labs had taken the quality performance of our “old reliable” dewar tanks for granted. For example, it was not uncommon to be dipping daily into a readily available dewar to facilitate immediate LN2 lab use needs, which of course impacts the accuracy of weekly tracking of a tanks’ evaporation rate. The latter event should be prohibited and an alternative tank (e.g., old retired) should be filled for strictly dedicated “LN2 usage” in the lab. Additional, such a filler tank can be used as an ‘emergency back-up tank’, if ever needed to temporarily store the entire contents of a failing tank.
Overall, the most impactful realization gained from failure events is that we are all susceptible to complacency in our cryostorage quality control practices. Whether it involved dip-stick measurement variations, inconsistent filling practices, poor control & monitoring of shipments, simple failure to employ daily QC, unprepared emergency plan implementation or the lack of recognition to alarms and monitoring alerts, most of us were living at-risk. In short, we are each managing potentially severe liabilities every day. Surprisingly, many of our answers to concerns has involved some very basic acknowledgements and practical ideas: 1) storage tanks should be maintained in secure but highly visible areas; 2) twice daily physical inspections of each tank are important; 3) rigorous attention to detail (e.g., weekly pre-fill measurements, alarms) is informative and patterns should be tracked; 4) monitoring systems and alarms are critical safeguards; 5) record keeping must be thorough and complete; 6) the delegation of QC duties may need to be rotated to reduce complacency and provide internal auditing; and 7) cryostorage tanks can and will fail. The last point is the most important and is best addressed by the first four points. Several investigator over the past two years (DA Kelk, KO Pomeroy, ML Reed, B LoManto, S Harris, WB Hazelrigg, ZP Michaelson and MC Schiewe; see 2019 References) have conducted vacuum failure experiments and have exhibited why the above measures (1-4) are so important (see Figure). In brief, their demonstrations have taught us that vacuum breeches lead to distinct visual events: firstly, an overt vaporization response around the cap and neck of the tank (within 30 sec); secondly, frosting and ice build-up evident on the cap (within 3 min); and thirdly, gradual ice and condensation accumulation on the exterior surface until warmed (>1 hour later). These observations are the immediate result to heating the inner tank and the creation of temperature gradients/changes between the inner and outer surfaces previously shielded by an insulating vacuum layer. These physical indicators are evident early on (beginning within minutes: * [see * in chart below] reduced external temperature around neck of tank) while changes in liquid volume and temperature are gradual, with critical changes in temperature (above -150°C) not occurring until nearly all LN2 has vaporized (as indicated by the change in the slope of the warming curve). It is possible that LN2 vapor storage tank may give us up to 12 hr less response time to a crisis, thus lending us critical information for risk assessment analysis, i.e., failure response time versus disease transmission. One has been repeatedly proven possible, while the other has not.
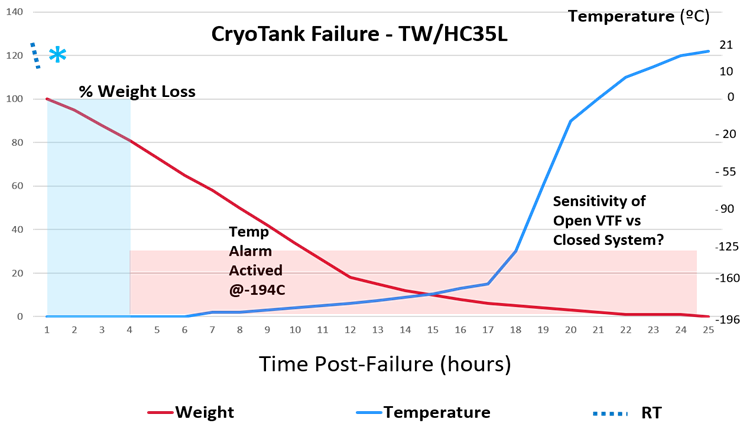
Figure: A series of 35L dewar tank failures (n=7) were conducted under continuous weight, temperature and video observation. This graph presented at ASRM2019 (Schiewe, PGC04) conveys a series of mean responses when the vacuum is breeched externally at the outer port or internally at the base by drilling.
Beginning with Dawn Kelk’s eye-opening tank failure demonstration experiment at ASRM 2018, these studies have dramatically shown us the power of observation (i.e., LIFES: leakage, ice, frost, evaporation/condensation, and sounds), the time periods we have for emergency response and the importance of monitoring documentation. The publicized failure events also elicited a substantial recall of various USA manufactured dewar tanks due to potentially incomplete welds (e.g., 2018 Chart Recall). Unfortunately, additional recalls by CHART (the primary manufacturer of all LN2 dewar tanks) have since occurred. Whether these recalls are valid or preventative is unclear, but it is yet another clear reminder of the importance of strict quality management practices. Besides routine QC measures, all tanks should undergo validation/qualification processes for installation (IQ), pre-use operation (OQ) and ongoing performance (PQ). In brief, a couple key points to consider, include: IQ- if a tank has a dent in it? That might be a significant defect or could be considered an indication of an unintentional drop test that challenged the strength of welds (i.e., a QC practice recommended by some experts), the significance of which would depend on OQ; OQ- fully evaluates a new tank’s evaporation rate potential (Evap) prior to use, including a possible “stress test”. If it loses nitrogen too quickly on a weekly basis, or when challenged by test conditions, then retire the tank, returning it to the manufacturer due to poor weld quality or insufficient vacuum; and PQ – weight has long been used by sperm cryobanks to assess dry-shipper tank reliability. Interestingly, it took the recent catastrophe(s) to stimulate us, as scientists, to be logical and realize the potential value of weight as an objective measure of LN2 level and volume change in Dewar tanks (Michaelson et al., 2019).
Recent, real and experimental, cryostorage tank failures have motivated all us to be more diligent in our cryobank quality management practices. The old reliable negative pressured, double layered, insulated tank systems are susceptible to malfunction. Today, we must safeguard our liabilities and question product quality, as well as new tank systems. Besides implementing effective monitoring and alarm systems, we need to use our scientific brains to question and innovate better ways (e.g., remote weight and external temperature monitoring) to anticipate and respond more efficiently to a tank failure. A mind is a terrible thing to waste, so do not be complacent in any aspect of crystorage management. This communication has only touched on physical failure management, while communication and documentation practices with patients represents an equally important subject to be addressed in a future Newsletter publication.
References:
Michaelson ZP, ST Bondalapati, S Amrane, et al. 2019. Early detection of cryostorage tank failure using a weight-based monitoring system. JARG; 36(4):655-660.
Moutos CP, R Lahham, JY Phelps. Cryostorage failures: A medicolegal review. J Asst. Repro. Genet. 2019; 36(6):1041-48. Pomeroy K. Liquid nitrogen storage tank failure: Can we improve the current system? Fertil Steril. 2018; retrieved from https://www. fertstertdialog.com.
Pomeroy KO, ML Reed, B LoManto, et al. 2019. Cryostorage tank failures: temperature and volume loss over time after induced failure by removal of insulative vacuum. JARG; 36(11):2271-2278.
Schiewe MC, M Freeman, JB Whitney, et al. 2019. Comprehesive assessment of cryogenic storage risk and quality management concerns: best practice guidelines for ART labs. JARG; 36(1):4-15.
Schiewe MC. Preventing cryostorage failure. In: Cryostorage Management and the Lost Art of ART (MC Schiewe, T Pool, D Fish, Faculty). Am Soc Reprod Med 52nd Ann Pre-Congress, PC04 2019. Pp.70-79.
Tomlinson M, Morroll D. Risks associated with cryopreservation: a survey of assisted conception units in the UK and Ireland. Hum Fertil (Camb). 2008;11:33–42
Pomeroy K. Liquid nitrogen storage tank failure: Can we improve the current system? Fertil Steril. 2018; retrieved from https://www. fertstertdialog.com.
Schiewe MC, MF Freeman, JB Whitney, MD VerMilyea, A Jones, M Aguirre, C Leisinger, G Adaniya, N Synder, R Chilton, EJ Behnke. Comprehensive assessment of cryogenic storage risk and quality management concerns: Best practice guidelines for ART labs. J Asst Reprod Genet. 2019; 36(1):5-14.
Pomeroy KO.ML Reed, B LoManto, SG Harris, WB Hazelrigg, DA Kelk. Cryostorage tank failures: Temperature and volume loss over time after induced failure by removal of insulative vacuum. J Asst Reprod Genet. 2019:In press.
Michaelson ZP, ST Bondalapati, S Amrane, R.W. Prosser, D.M. Hill, P. Gaur, M. Recio, D.E. Travassos, M.D. Wolfkamp, S. Sadowy, C. Thomas, E.J. Forman, Z. Williams. Early detection of cryostorage tank failure using a weight-based monitoring system. J Asst Reprod Genet. 2019;36:655-60.
Disclaimer: The content and views presented in this article are those of the author and do not necessarily reflect those of the American Association of Bioanalysts (AAB) or the AAB College of Reproductive Biology (CRB). The information is not necessarily exhaustive of the subject matter, and laboratory professionals and all other individuals should review and consider other relevant materials, and professional opinions and advice, on the subject matter before making appropriate decisions.
Digital QA – The future of ART Lab Management ⇒CRB News Articles
06/24/2020
Vol 10, No. 1
06/24/2021
From The President
06/24/2021
Meeting Committee
06/24/2021
Legislative Committee
06/24/2021
Credentialing and Membership Committee - Survey on Impact of COVID-19
06/24/2021
Publication Committee
06/24/2021
2021-2022 CRB Officers and Board Members
06/24/2021
Earn ABB/PEER CE Credit Attending Select 2021 ASRM Virtual Post-Graduate Courses
06/24/2021
2022 CRB Symposium, May 11-14
06/24/2021
Preventing Cryostorage Failure: What Have We Learned?
06/24/2021
Digital QA - The Future of ART Lab Management
06/24/2021
Board Certification for Andrology and Embryology Laboratory Professionals
CRB Standing Rules - Log in to view



